Page 168 -
P. 168
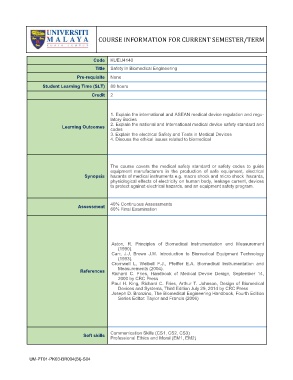
COURSE INFORMATION FOR CURRENT SEMESTER/TERM
Code KUEU4140
Title Safety in Biomedical Engineering
Pre-requisite None
Student Learning Time (SLT) 80 hours
Credit 2
1. Explain the international and ASEAN medical device regulation and regu-
latory Bodies
2. Explain the national and International medical device safety standard and
Learning Outcomes
codes
3. Explain the electrical Safety and Tests in Medical Devices
4. Discuss the ethical issues related to biomedical
The course covers the medical safety standard or safety codes to guide
equipment manufacturers in the production of safe equipment, electrical
Synopsis hazards of medical instruments e.g. macro shock and micro shock hazards,
physiological effects of electricity on human body, leakage current, devices
to protect against electrical hazards, and an equipment safety program.
40% Continuous Assessments
Assessment
60% Final Examination
Aston, R. Principles of Biomedical Instrumentation and Measurement
(1990).
Carr, J.J, Brown J.M. Introduction to Biomedical Equipment Technology
(1993).
Cromwell L, Weibell F.J., Pfeiffer E.A. Biomedical Instrumentation and
Measurements (2004).
References
Richard C. Fries, Handbook of Medical Device Design, September 14,
2000 by CRC Press
Paul H. King, Richard C. Fries, Arthur T. Johnson, Design of Biomedical
Devices and Systems, Third Edition July 29, 2014 by CRC Press
Joseph D. Bronzino, The Biomedical Engineering Handbook, Fourth Edition
Series Editor: Taylor and Francis (2006)
Communication Skills (CS1, CS2, CS3)
Soft skills
Professional Ethics and Moral (EM1, EM2)
UM-PT01-PK03-BR004(BI)-S04