Page 170 -
P. 170
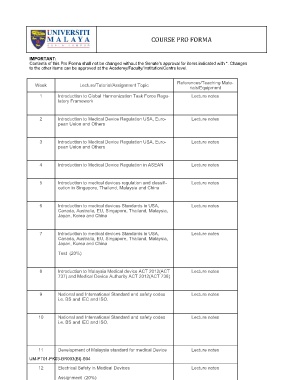
COURSE PRO FORMA
IMPORTANT:
Contents of this Pro Forma shall not be changed without the Senate’s approval for items indicated with *. Changes
to the other items can be approved at the Academy/Faculty/Institution/Centre level.
References/Teaching Mate-
Week Lecture/Tutorial/Assignment Topic
rials/Equipment
1 Introduction to Global Harmonization Task Force Regu- Lecture notes
latory Framework
2 Introduction to Medical Device Regulation USA, Euro- Lecture notes
pean Union and Others
3 Introduction to Medical Device Regulation USA, Euro- Lecture notes
pean Union and Others
4 Introduction to Medical Device Regulation in ASEAN Lecture notes
5 Introduction to medical devices regulation and classifi- Lecture notes
cation in Singapore, Thailand, Malaysia and China
6 Introduction to medical devices Standards in USA, Lecture notes
Canada, Australia, EU, Singapore, Thailand, Malaysia,
Japan, Korea and China
7 Introduction to medical devices Standards in USA, Lecture notes
Canada, Australia, EU, Singapore, Thailand, Malaysia,
Japan, Korea and China
Test (20%)
8 Introduction to Malaysia Medical device ACT 2012(ACT Lecture notes
737) and Medical Device Authority ACT 2012(ACT 738)
9 National and International Standard and safety codes Lecture notes
i.e. BS and IEC and ISO.
10 National and International Standard and safety codes Lecture notes
i.e. BS and IEC and ISO.
11 Development of Malaysia standard for medical Device Lecture notes
UM-PT01-PK03-BR003(BI)-S04
12 Electrical Safety in Medical Devices Lecture notes
Assignment (20%)